In this post, I am sharing FSC 1st Year Chemistry Chapter 7 Solved Exercise Notes PDF for the students of Intermediate Part 1. Name of this chapter is Thermochemistry. Students of Class 11 can download Thermochemistry Solved Exercise notes from this post. the post FSC 1st Year Chemistry Chapter 7 Solved exercise PDF contain 31 pages. I have already posted 1st Year Chemistry Complete Notes in PDF format.
11th Class Chemistry Chapter 7 Thermochemistry solved exercise PDF Download
What is the Difference Between internal energy and enthalpy?
| Internal Energy | Enthalpy |
| It is sum of kinetic energy and potential energy of the system. | It is sum of internal energy and product of pressure and Volume of the system |
| It is represented by ‘E’ | It is represented by ‘H’ |
| Mathematically it is E= K.E + P.E | Mathematically it is H = E+ PV |
| Its unit are joule or calorie | Its units are kilo joule or kilo calorie |
Explain that burning of a candle is a spontaneous process.
The process that makes place on its own is called spontaneous process. A process will also called spontaneous, if it needs energy to start with, but once it is started, then it proceed on its own.
A candle does not burn in air on its own; a spark initiates the burning of candle. Once it start burning, then the reaction proceed spontaneously to completion.
What is the first law of thermodynamics? How does it explain that
- qv = DE (ii) qp = DH
First law of Thermodynamics:
It stated that:
“Energy can neither be created nor be destroyed but it can be changed from one form to another”
OR
“ Internal energy change of the system (DE) is equal to the sum of heat evolved or absorbed (q) and work sone by oron the system.
Mathematically’
DE = q + w
DE = q +PDV
- qv = DE
Concider the heat supplied to the system at constant volume
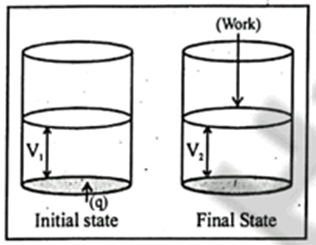
DE = q +PDV (at constant volume DV = 0 and PDV=0)
So, DE = 0
At constant volume Heat supplied is equal to internal energy change
- qp = DH
For this, consider the enthalpy change at constant pressure. Enthalpy is the sum of internal energy and product of pressure and volume as
H = E. PV
Enthalpy change will be:
DH =DE+ D(PV)
DH =DE+PDV + DPV (since P= Constant DP=0 and DPV)
DH =DE+PDV
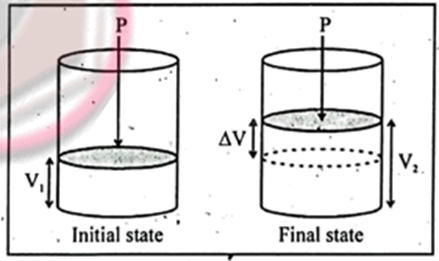
For liquid and solid
DV=0 and PDV=0
So DH = DE
For Gass; (since DV is not equal to 0)
DH = DE+ PDV
Put the value of DE = q – w
When heat is supplied at constant pressure, work is done by the system
So w = – PDV
DE = q – PDV
DH = q – PDV + PDV
DH = qp Heat supplied to the system at constant pressure is used to do work as well as in increasing the internal energy of the system. That’s why it is called as enthalpy.
Relevent Notes
1st Year Chemistry Chapter 1 solved Exercise PDF
11th Chemistry Chapter 2 solved Exercise PDF
1st Year Chemistry Chapter 3 solved Exercise PDF
11th Class Chemisrty Chapter 4 Solved exercise PDF
1st Year Chemistry Chapter 6 solved Exercise PDF
FSC Part 1 Chemistry Chapter 8 solved Exercise PDF
FSC 1st Year Chemistry Chapter 9 solved Exercise PDF
11th Class Chemistry Chapter 10 Solved Exercise PDF